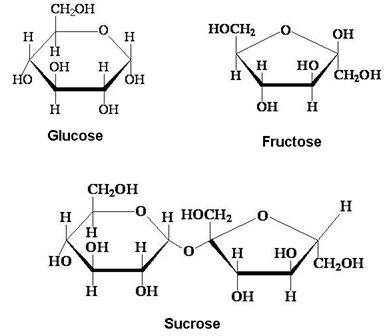
Disaccharides
Disaccharides:-
These are the carbohydrates which give two units of monosaccharides on hydrolysis. Some examples are: Sucrose, Lactose, Maltose.
C12H22O11 + H2O C6H12O6 + C6H12O6
Sucrose α-D-Glucose β-D-Fluctose
C12H22O11 + H2O C6H12O6 + C6H12O6
Lactose β-D-Glucose α-D-Glucose
C12H22O11 + H2O 2 C6H12O6
Maltose α-D-Glucose
This implies that they are formed by the condensation of two monosaccharides. The linkage which holds monosaccharide units in a disaccharide (or polysaccharide) molecule is called Glycoside Linkage. e.g.
(i) Sucrose:- In sucrose, α-D-Glucose and β-D-Fructose condense together: (C1 – C2 linkage).
(ii) Lactose:- In Lactose, Glactose unit and glucose unit condense together (C1 – C4 linkage).
(iii) Maltose:- In Maltose two units of α-D-Glucose condense together (C1 – C4 linkage)
The Disaccharides which involve the carbonyl group in the glycoside linkage is called non reducing sugar e.g. sucrose and the disaccharides which involve no carbonyl group and is free are called reducing sugars e.g. Maltose and Lactose.
 SureDen
SureDen