Ionisattion Energy
Ionisation Energy / Ionisation Enthalpy (I.E.)
It is amount of energy required to remove most loosely bound electron from gaseous atom to form gaseous cation
Example
It is expressed in Kj/mole , Kcal/mole , en/mol , en/atom
Successive Ionisation energy are the energies required to remove Ist, IInd and IIIrd electron one by one.
I.E3 > I.E2 > I.E1
Second Ionisation energy. is always greater than first Ionisation energy because after the loss of one electron, the size decreases and remaining electrons are tightly held to the nucleus so more energy is required to remove them.
Factors affecting Ionisation Energy.
(1) Size:-Smaller the size of atom, higher is the Ionisation Energy.
(2) Effective Nuclear Charge:- greater the electro negative charge greater is Ionisation Energy.
(3) Penetration Effect (Closeness to the Nucleus):- greater this effect, greater Ionisation Energy.
Example- S > P > D > F ( decreasing order of penelvation effect.
IE(s) > IEp > IE(d) > IEf. for the same shell.
(4) Half filled / Fully filled configuration:- There type of configurations are extra – stable ionisation Energy are tightly held to the nucleus, so greater is the Ionisation Energy.
(5) Screening Effect / Shielding Effect:- greater the Screening Effect, grater the Ionisation Energy. Electro Nuclear charge Decreases for outermost Shell.
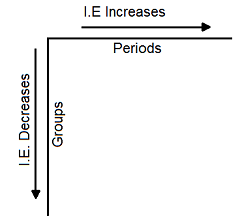
Trend of Ionisation Energy along the Periods and Groups
Another Exceptional Case In Periods
Ionisation Energy of Nitrogen > Ionisation Energy of Oxygen
Ionisation Energy of phosphorous > Ionisation Energy of Sulpher.
Same Reason 7N - 1s2 2s2 2p3
8O - 1s2 2s2 2p4
Nitrogen has half filled p orbital , which is extra stable so more energy is required to remove last eΘ .
Important Question and Answer
Q(1) First Ionisation Energy of Na is < that of mg. but second Ionisation Energy of Na is higher than that of Mg?
Ans. 71Na- 1s2 2s2 2p6 3s1 Mg- 1s2 2s2 2p6 3s2
I case:- Mg has fully filled extra stable configuration. So more energy is required
II case:- Configuration will be
Na+ - 1s2 2s2 2p6 Mg+ - 1s2 2s2 2p6 3s1
Now Na+ has fully filled extra stable configuration. So more energy is needed to remove outermost election
Q. Why isolated gaseous atom is taken in determination of ionisation Energy?
Ans. eΘ is always lost from the gaseous isolated atom not in the liquid / solid state and also for the compassion purposes, eΘ lost from isolated gaseous atom.
 SureDen
SureDen