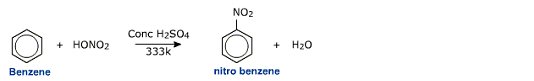
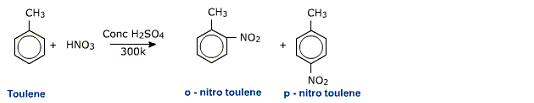
Laboratory Preparation of Nitrobenzene
Laboratory preparation of nitrobenzene
Aromatic compounds can be directly nitrated using a mixture of concentrated nitric acid and sulphuric acid.
This reaction is an example of electrophilic substitution of benzene. The electrophile is a nitronium (NO+2)
ion.
The nitronium ion attacks to the benzene ring resulting in the formation of a carbocation. Electron releasing substituents like -CH3, -OCH3, -OH, -NH2 etc activate the ring and stabilize the carbocation while electron withdrawing groups like -NO2, -CN, -SO3H, -X activate the carbocation. The nitrating mixture of conc H2SO4 and conc HNO3 con nitrate even deactivated compounds like nitrobenzene under refluxing conditions to get m-dinitrobenzene. For nitration of activated aromatic systems like phenol and then ether derivatives, even milder conditions can be used.
 SureDen
SureDen