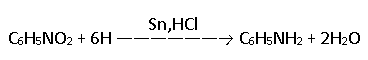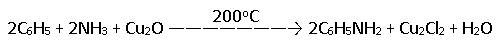Laboratory Preparation of Aniline
Laboratory preparation of aniline
Aniline was first prepared by Unverdorben by dry distillation of indigo. In the laboratory, it can be prepared by the reduction of nitrobenzene with tin and hydrochloric acid(HCL).
Aniline produced combines with H2SnCl6 (SnCl4 + 2HCl) to form a double salt.
2C6H5NH2 + SnCl4 + 2HCl —→ (C6H5NH3)2 SnCl6
Double salt
From double salt, aniline is obtained by treating with conc. caustic soda solution.
(C6H5NH3)2SnCl6 + 8NaOH —→ 2C6H5NH2 + 6NaCl + Na2SnO3 + 5H2O
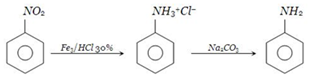
On a commercial scale, aniline is obtained by reducing nitrobenzene with iron filings and hydrochloric acid.
Aniline is also obtained on a large scale by the action of amine on chlorobenzene at 200°C under 300-400 atm pressure in presence of cuprous catalyst.
All the Best.
 SureDen
SureDen