Chemical Properties of Aromatic Nitrocompounds
Chemical properties of aromatic nitrocompounds
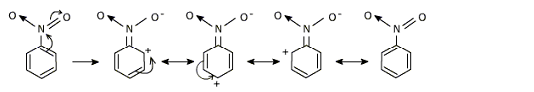
The nitro group strongly deactivates the benzene ring towards electrophilic substitution. Nitro group is electron withdrawing group and thus causes electron deficiency at ortho and para positrons as is clear from the resonating structures of nitro benzene.
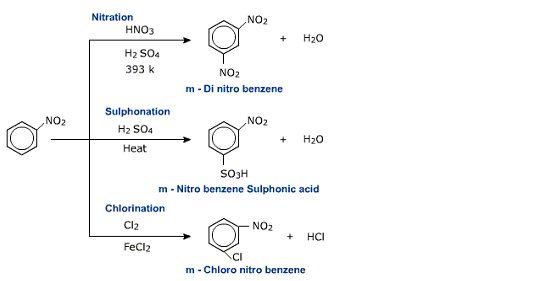
Since the meta positron is relatively rich in electron density compared to ortho and para positrons electrophilic attack is more likely to occur at meta position. Thus the -NO2 group is meta directing as far as electrophilic ring substitution is concerned.
Electrophilic substitution reaction
Influence on the reaction of other functional group
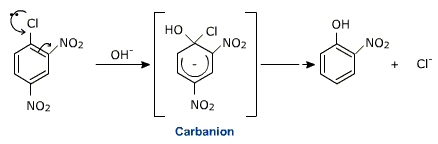
The presence of nitro group facilitates nucleophilic substitution of otherwise unreactive aromatic halides by stabilizing the intermediate carbanion.
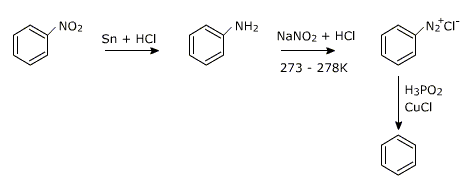
Reduction of nitrobenzene
The nitro group can be removed from an aromatic ring via the following steps:
i) Reduction of nitro group to amine
ii) Diazotization of amine with HNO2
iii) Reductive removal of the diazonium group using sodium borohydride or hypophosphorus acid/Cu+ mixture.
 SureDen
SureDen