Excess Pressure
Due to the property of surface tension a drop or bubble tries to contract and so compresses the matter enclosed. This in turn increases the internal pressure which prevents further contraction and equilibrium is achieved. So in equilibrium the pressure inside a bubble or drop is greater than outside and the difference of pressure between two sides of the liquid surface is called excess pressure. In case of a drop excess pressure is provided by hydrostatic pressure of the liquid within the drop while in case of bubble the gauge pressure of the gas confined in the bubble provides it.
Excess pressure in different cases is given in the following table :
|
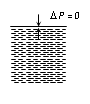
Plane surface |
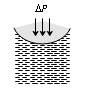
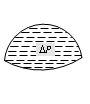
Concave surface |
|
DP = 0
|
|
|
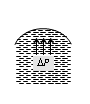
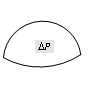
Convex surface |
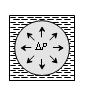
Drop |
|
|
|
|
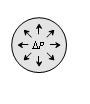
Bubble in air |
Bubble in liquid |
|
|
|
|
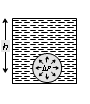
Bubble at depth h below the free surface of liquid of density d |
Cylindrical liquid surface |
|
|
|
|
Liquid surface of unequal radii |
Liquid film of unequal radii |
|
|
|
 SureDen
SureDen