Surface Tension
Surface Tension (S.T.) ⟶ Surface tension of a liquid defied as the force acting at slight angles to the surface along one centimeter length of the surface.
Units of surface tension are dynes cm-1 or Nm-1
Surface energy ⟶ is defined as the work is ergs required to be alone, to increase or external the surface area by square cm.
Units of surface energy are ergs cm-2 Jm-2 surface tension and surface energy is same thing
Imp. Results ⟶
(1) The small drops of water are spherical because of surface tension, they try to acquire min. surface area.
(2) Fire polishing of glass ⟶ on heating glass edges. Glass melts and takes up sounded shape.
(3) Water rises in capillary due to surface tension.
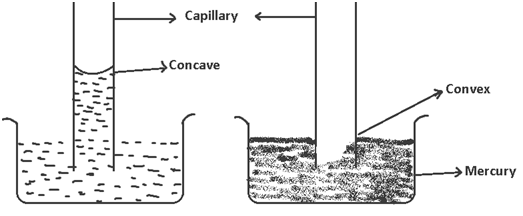
(4) The liquids which not the glass, will rise up is capillary and have concave meniscus e.g. water this is because, their adhesive forces (force of attraction bet. liquid and glass) are stronger than cohesive forces (force of attraction bet. liquid- liquid molecules i.e. of same substance).
The liquids which don’t wet glass, show fall is capillary e.g. Hg because their cohesive forces are stronger than adhesive forces & they show convex meniscus.
⟶ Greater the intermolecular force of attraction between liquid molecule, increases is surface tension.
⟶ With the increase of temperature, surface tension decreases become kinetic energy of molecules increase
⟶ Its dimensions are J/m-2
 SureDen
SureDen