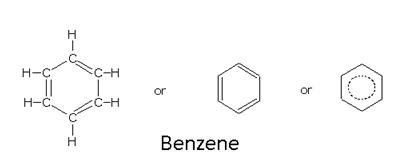
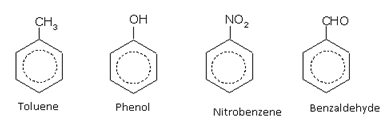
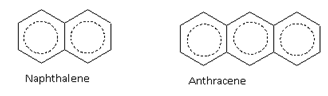
Nomenclature of Aromatic Hydrocarbons
Common names of organic compounds
Common nomenclature
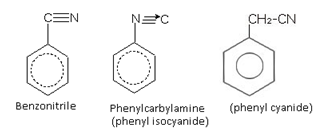
In the common system, cyanides are named by any of the following methods:
(i) By using suffix cyanide after the name of alkyl and aryl group.
or
(ii) By adding the suffix o-nitrile in place of ic - acid in the common name of the corresponding acid produced by the hydrolysis of the cyanide compound.
For e.g., CH3 CN on hydrolysis gives CH3 COOH i.e., acetic acid.
So the common name of the compound is methyl cyanide or Acetonitrile as derived from acetic acid.
The aromatic compounds may have a side-chain or a functional group attached directly to the ring. For example,
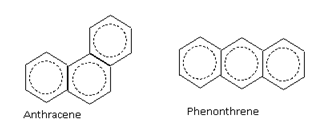
The aromatic compounds may also contain more than one benzene rings fused together
There are two classifications of aromatic compounds.
Nuclear Substituted Compounds
When the functional group or any substituent, in aromatic compounds is directly attached to the benzene ring, it is a called nuclear substituted compound. Such compounds are named as the derivatives of benzene under the IUPAC system. However, the common names of many such compounds are retained by IUPAC.
Sidechain Substituted Compounds
Aromatic compounds where the functional group is present in the sidechain of the ring are called sidechain substituted compounds. Sidechain substituted compounds are named as the phenyl derivatives of the corresponding aliphatic compounds.
Naming Benzene Derivatives
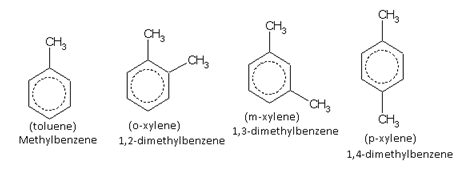
Only one kind of monosubstituted derivatives are possible in benzene rings as all six hydrogen atoms are equivalent. For example, there is only one toluene. It does not matter where the methyl group is attached because all the following arrangements are equivalent.
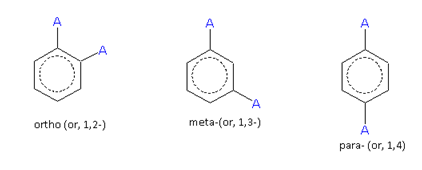
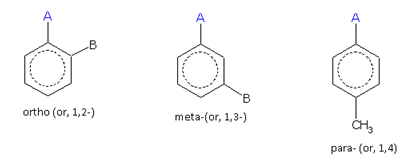
When two same or different monovalent substituents, are present on a benzene ring, the following three arrangements are possible.
For the same substituent (A)
For different substituents (A and B)
These arrangements are named as follows:
The compound containing the two groups on the adjacent sites is called 'ortho'; it is denoted as 'o-'. In the IUPAC system, the ortho position is designated as 1,2-.
The compound containing the two groups on alternate sites is called 'meta': it is denoted as 'm-'. In the IUPAC system,the meta position is designated as 1,3-.
The compound containing the two groups diagonally opposite to each other is called 'para': denoted as 'p-'. In the IUPAC system, the para position is designated as 1,4-.
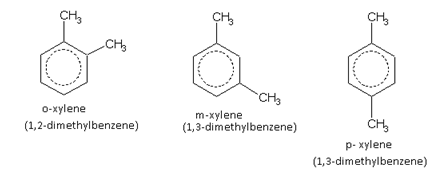
For example, the three xylenes are named as,
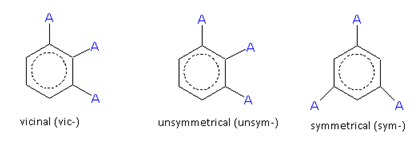
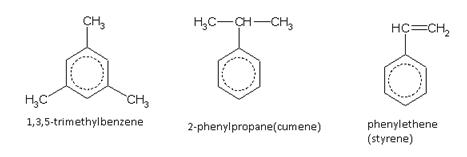
In the case of trisubstituted derivatives, the nature of the substituted groups determine the number of arrangements. When the three substituent groups are identical (say, A), three arrangements are possible. These are termed as follows.
For a trisubstituted product, if the two substituents are identical and the third different, then six products are possible. When all the three groups are different, ten products are possible. Since, naming each individual compound is not possible, it was found convenient to indicate the position of any substituent by the numeral indicating the serial number of the carbon atom in the ring, to which that substituent is attached.
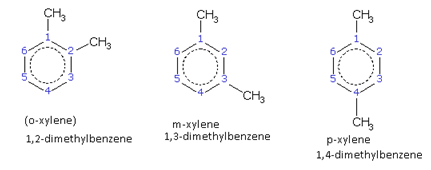
Numbering the Carbon Atoms in the Ring
The numbering of carbon atoms in the ring (or nucleus) is done as follows.
When there is only one substituent on the ring, there is only one compound possible. Thus, numbering of the carbon atoms of the nucleus does not arises.
If there are two or more substituents, then numbering is in the alphabetical order of the substituents on the carbon atoms. The prefixes such as 'di', 'tri', 'cyclo', 'iso', etc., are ignored while arranging the substituents alphabetically.
When two or more functional groups are present, then the principal functional group is assigned the number 1. The order of priority of the functional groups is the same as done for aliphatic polyfunctional compounds.
For the sake of convenience, the ring is oriented in such a way that position 1 is at the top and numbering is done in a clockwise or anticlockwise manner whichever gives lower numbers to the other substituents.
This is illustrated through the following example. The IUPAC names are written in bold letters.
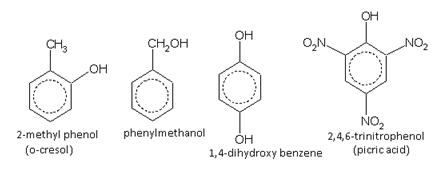
Names of some typical aromatic compounds are given below:
Aromatic hydrocarbons (arenes)
Halogen derivatives
Hydroxy derivatives:
Phenols and aromatic alcohols
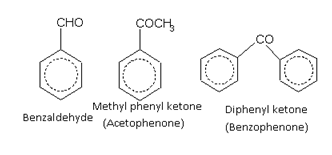
Aldehydes and Ketones
Nuclear substituted
Carboxylic acids
Nuclear substituted
Acid derivatives
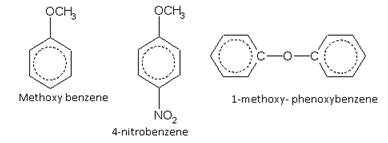
Alkoxy derivatives
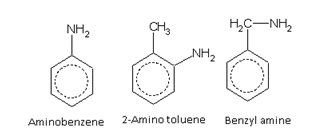
Amines
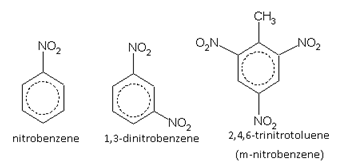
Nitro derivatives
Nitriles and Carbylamines
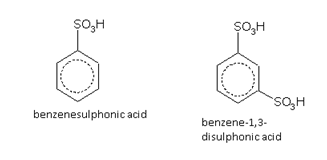
Sulphonic acids
Aromaticity
Aromatic compounds are those, which resemble benzene in chemical behavior. These compounds contain alternate double and single bonds in a cyclic structure. They undergo substitution reactions rather than addition reactions. This characteristic behavior is called aromatic character or aromaticity the criteria for which are as follows:
Contains a cyclic cloud of delocalized p electrons above and below the plane of the molecule.
Electrons cloud must contain a total of (4n+2) a electrons, where p is an integer equal to 0,1,2,3 ...........
This is known as 'Huckel rule' according to which the aromatic compounds have delocalized electron cloud of p electrons of 2 or 6 or 10 or 14 electrons.
For example, benzene (6p electrons), naphthalene (10p electrons) and anthracene (14p electrons) are aromatic compounds.
6p electrons 10p electrons 14p electrons
Similarly, cyclopentadienyl anion and cycloheptatrienyl cation (tropylium ion) are also aromatic because these contain 6p electrons.
6p electrons (Tropylium ion) 6p electrons
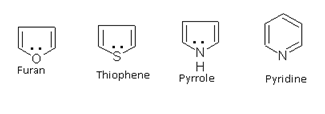
Heterocyclic compounds such as pyrrole, furan, thiophene and pyridine also behave as aromatic because all have 6p electrons.
 SureDen
SureDen