Short Notes
Biomolecules
Biomicromolecules:
- All living organism, from microbes to mammals, are composed of chemical substance.
- These chemical substances can be organic or inorganic. Chemical analysis is performed to find out chemical composition of a living cell.
- Water is the most abundant chemical compound in living body.
- Most of the organic compounds are found in acid-soluble fraction.
- Inorganic compounds such as sulphate, etc. are also found in acid-soluble fraction.
Organic compounds:
- All carbon compounds obtained from living tissues are known as organic biomolecules. These are two types:
- Micromolecules bio-micromolecules
- Macromolecules bio-macromolecules
Micromolecules Bio-micormolecules:
These are the organic compounds with molecular weights less than one thousand Dalton. Classified as amino acids, sugars, nucleotide bases, etc.
These are also known as primary metabolites.
Secondary metabolite on the other hand are compounds other than primary metabolites. These are commonly found in plants, fungi and microbes. For example- Alkaloids, flavonoids and essential oils etc. are secondary matabolites.
Amino acids:
- These are organic compounds containing an amino group, carboxyl group, carboxyl group, hydrogen and a variable functional group R.
- Common structure of an amino acid is based on the R group, there are many amino acids, but only 20 types of amino acids, but only 20 types of amino acids constitute proteins.
-
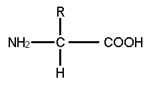
- Simplest amino acid is glycine, which contains hydrogen as the R group. Structure of glycine is
-
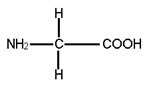
- Amino acids can be acidic when carboxylic group is more, e.g. glutamic acid, basic when amino group is more e.g. lysine or neutral when both carboxylic and amino group are same in number, e.g. valine.
Fatty acids:
Organic compounds containing a carboxyl group attached to an R group. R group is variable and contains 1 to 19 carbones.
Fatty acids can be saturated without C=C double bond or unsaturated with one or more C=C double bonds.
These are water insoluble copounds.
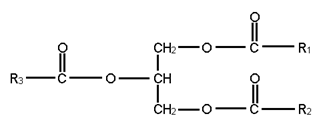
Structure of fatty acid palmitic acid.
CH3 – (CH2)14 – COOH
Fatty acids are esterified with glycerol to form monoglycerides, diglycerides and triglyceride is
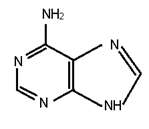
Nitrogen bases
These are organic compounds containing heterocyclic rings.
Adenine, guanine, cytosine, uracil and thymine are nitrogenous bases.
Structure of ademine is
When attached to sugar, these are known as nucleosides.
When a phosphate group is also attached along with sugar, these are known an nucleotides.
Bio-macromolecules:
Organic compounds with molecular weight in the range of ten thousand Daltons and more are known as bio-macromolecules. Lipids are an exception.
These molecules are found in acid insoluble fraction.
These are classified as proteins, polysaccharides, nucleic acids, etc.
- Proteins:
- Proteins are linear chains of amino acids linked by peptide bonds, hence also known as polypeptides.
- A peptide bond is formed between the carboxyl group of one amino acid and the amino group of next amino acid.
- Essential amino acids obtained through diet or food are part of dietary proteins.
- Functions of proteins:
- To transport nutrients
- Fighting infections
- Act as hormones and enzymes
- Collagen is the most abundant protein in the animals whereas RuBisCO Ribulos e bisphosphate Carboxylase – Oxygenase is the most abundant protein universally.
- Structures of Proteins:-
- Primary structure is the linear chain of amino acids.
- Secondary Structure is the helical folded structure.
- Tertiary Structure is the Three-dimensional view of protein.
- Quaternary structure is the assembly of more than one polypeptide.
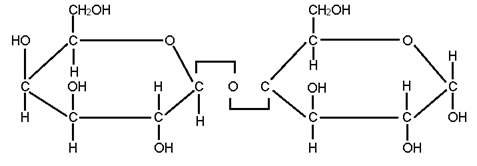
- Polysaccharides:
- Polysaccharides are long chains of monosaccharides.
- In a plysaccharde, the individual monosaccharides are joined by glycosidic bonds. Structure of glycosidic bond is
- Structure of glycosidic bonds is
- Cellulose is a polysaccharide consisting of only one type of monosaccharide- glucose. Therefore, cellulose is a homopolymer or simple polysaccharide.
- Similary, inulin is a polymer of fructose.
- Complex polysaccharides have building blocks, amino-sugars and chemically mocdified sugars such as glucosamine, N-acetyl galactosamines, etc. Hence, they are heteropolymers.
- Chitin is an example of complex polysaccharide. It forms the exoskeleton of arthropods and cell wall of fungi.
Nucleic acids:
- Nucleic acids are polynucleotides.
- A nucleic acid containing deoxyribose sugar is DNA and that with ribose sugar is RNA.
- Bond formed between phosphate of one Nucleotide and hydroxyl group of sugar present in other nucleotide is known as phosphodiester bond.
Nature of Bonds Linking Monomers in a Polymer:
Peptide Bond:
- It links amino acids in a polypeptide chain.
- This bond is formed when the carboxyl group of one amino acid reacts with the amino group of the next amino acid, with the elimination of water moiety dehydration.
Glycosidic Bond:
- It links two nucleotides to form nucleic acids.
- This bonds is formed between the phosphate and hydroxyl groups of sugar.
- All chemical reactions occurring in a living organism are together known as metabolism.
- Biosynthetic pathways where simple structure form complex structures are known as anabolic pathways.
- Degradation pathways where complex structures break to form simple structures are known as catabolic pathways.
- Anabolic pathways consume energy whereas catabolic pathways lead to the release of energy.
- Energy is liberated in the form of ATP Adenosine triphosphate.
- The rate of metabolic conversions anabolism or catabolism is affected by catalysts called enzymes.
Primary and secondary metabolites:
- Primary metabolites - Intermediates or products of metabolism directly involved in growth, development and reproduction.
Example: Fatty acids, amino acids, etc.
- Secondary Metabolites – Intermediates or products of metabolism not involved directly in growth, development and reproduction.
Example: Pigments such as carotenoids, toxins, drugs and essential ois.
Enzymes:
General Features:
- Almost all enzymes are proteins. Those RNA which can catalyze their own biochemical reactions are called ribozymes.
- The site of an enzyme at which a substrate fits is called its active site. These can catalyze reactions at a high rate.
- Example:
-

- Activation energy is the energy required to achieve the transition state.
- Enzymes are divided into 6 classes based on the type of reaction they catalyzeoxido-reductases, hydrolases, transferases, lyases, isomerases and ligases.
Cofactors:
- The protein part of an enzyme is called apoenzyme.
- Cofactors are non-protein constituents bound to the enzyme. These make the enzyme catalytically active.
- Cofactors can be classified in three categories.
- Prosthetic groups – These are organic compounds which tightly bind to the apoenzyme. Example: Haem.
- Coenzymes – These are organic molecules which unite with the apoenzyme only during the course of reaction. Example: NAD.
- Metal ions – Example: Zinc acts as cofactor for carboxypeptidase.
Factors Affecting enzyme Activity:
- Enzymes do not start a reaction, but help in accelerating it.
- Enzymes affect the rate, But not the direction of a biochemical reaction.
- Mist of the enzymes have high turnover number. Turnover number of an enzyme is the number of molecules of a substance that is acted upon by an enzyme per minute. High turnover number of enzymes increases the efficiency of reaction.
- Enzymes are specific in action.
- The temperature and pH at which an enzymes shows the maximum activity called the optimum temperature and optimum pH respectively.
- The activity of an enzyme declines both above and below the optimum value. Enzymes show maximum activity at an optimum pH of 6 – 8.
- The velocity of enzymatic reaction increases with increase in substrate concentration and then ultimately reaches a maximum velocity.
- A chemical which shuts off the enzyme activity is called inhibitor.
- When an inhibitor binds to the active site and competes with substrate for binding, it is called competitive inhibitor.
Nature of an enzyme action:
- The enzyme E first unites with the substrate S and forms an intermediate enzyme substrate complex ES.
- Then it undergoes an intermediate formation of enzyme product complex EP and finally it dissociates into its product P and enzyme regains its original form.
-
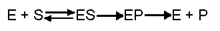
 SureDen
SureDen